Simple Definition Of Buffer Solution
A buffer is an aqueous solution containing a weak acid and its conjugate base or a weak base and its conjugate acid. Buffer systems are present in.
 Solubility Common Ion Effect Buffer Solution Buffer Solution Solubility Solutions
Solubility Common Ion Effect Buffer Solution Buffer Solution Solubility Solutions
Definition A buffer solution is one which resists changes in pH when small quantities of an acid or an alkali are added to it.

Simple definition of buffer solution. In other words a buffer is an aqueous solution of either a weak acid and its conjugate base or a weak base and its conjugate acid. Chemistry A mixture in which particles of one or more substances are distributed uniformly throughout another substance so that the mixture is homogeneous at the molecular or ionic level. They resist a change in pH upon dilution or upon the addition of small amounts of acidalkali to them.
Buffers do so by being composed of certain pairs of solutes. Buffer definition is - fellow man. Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications.
Either a weak acid plus its conjugate base or a weak base plus its conjugate acid. A buffers pH changes very little when a small amount of strong acid or base is added to it. Its pH changes very little when a small amount of strong acid or base is added to it.
Buffers usually consist of a weak acid and its conjugate base in relatively equal and large quantities. Buffers are solutions that resist a change in pH on dilution or on addition of small amounts of acids or alkali. The pH will remain essentially constant as long as the ratio of the concentrations of acids and bases are more or less constant.
A buffer may also be called a pH buffer hydrogen ion buffer or buffer solution. Buffer Solution Buffer systems have the ability to resist drastic changes in pH. A buffer solution more precisely pH buffer or hydrogen ion buffer is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or vice versa.
Its pH changes very little when a small amount of strong acid or base is added to it and is thus used to prevent a solution s pH change. A lot of biological and chemical reactions need a constant pH for the reaction to proceed. How to use buffer in a sentence.
Buffer Solution is a water solvent based solution which consists of a mixture containing a weak acid and the conjugate base of the weak acid or a weak base and the conjugate acid of the weak base. A buffer is a solution containing either a weak acid and its salt or a weak base and its salt which is resistant to changes in pH. A buffer solution is chemical solution which resists change to its pH or acidity.
A buffer solution is one in which the pH of the solution is resistant to small additions of either a strong acid or strong base. It is used to prevent any change in the pH of a solution regardless of solute. The particles in a solution are smaller than those in either a colloid or a suspension.
A solution that usually contains on the one hand either a weak acid as carbonic acid together with one of the salts of this acid or with at least one acid salt of a weak acid or on the other hand a weak base as ammonia together with one of the salts of the base and that by its resistance to changes in hydrogen-ion concentration on the addition of acid or base is useful in many chemical biological and technical processes. The pH will remain essentially constant as long as the ratio of the concentrations of acids and bases are more or less constant. A buffering agent is a weak acid or weak base that helps maintain the pH of an aqueous solution after adding another acid or base.
It is prepared by mixing a weak acid and a salt of its conjugate base or vice versa. The acetate ions shift the equilibrium depressing the ionization of the acetic acid. In this case the buffer solution is made of water acetic acid and sodium acetate.
The buffer capacity is numerically expressed to be equal with the minimum concentration of strong acid or strong base which causes the variation of buffers pH with one unit. Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications. In this case the buffer solution is made of water acetic acid and sodium acetate.
The pH of the solution changes very little when a small amount of strong acid or base is added to it. The buffer index can be defined as the differential ratio of the increase in the amount of strong acid or strong base added to pH variation. A buffer or buffered solution is one that resists a change in its pH when H or OH ions are added or removed owing to some other reaction taking place in the same solution.
It is a solution in water of a mixture of a weak acid or base and its salt. A buffer is an aqueous solution that consists of a mixture of a weak acid and its salt acid buffer or a weak base with its salt basic buffer. This does not mean that the pH of buffers does not change.
If you add an acid or a base to a buffered solution its pH will not change significantly. The acetate ions shift the equilibrium depressing the ionization of the acetic acid. Buffers are extremely useful in these systems to maintain the pH at a constant value.
A buffer is an aqueous solution that has a highly stable pH.
 Buffer Solution Preparation Of Buffer Solution Acidic Basic Buffer Buffer Action Buffer Solution Electron Configuration Solutions
Buffer Solution Preparation Of Buffer Solution Acidic Basic Buffer Buffer Action Buffer Solution Electron Configuration Solutions
 Buffer Solution Active Learning Instructional Sequence Part 1 Chemdemos
Buffer Solution Active Learning Instructional Sequence Part 1 Chemdemos
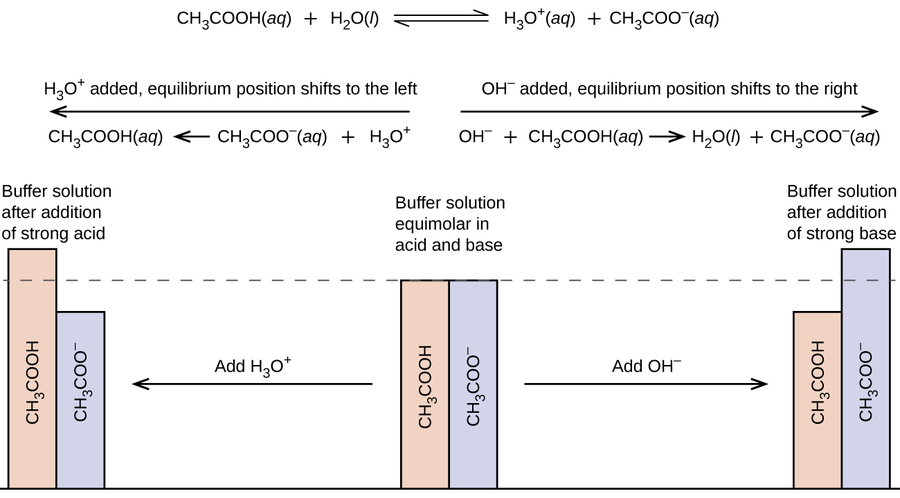 7 1 Acid Base Buffers Chemistry Libretexts
7 1 Acid Base Buffers Chemistry Libretexts
 Digital Kemistry Best Chemistry Animated Blogs What Is Buffer Solution Buffer Solution Chemistry Classroom Electron Configuration
Digital Kemistry Best Chemistry Animated Blogs What Is Buffer Solution Buffer Solution Chemistry Classroom Electron Configuration
 Buffer Solutions Study Material For Iit Jee Askiitians
Buffer Solutions Study Material For Iit Jee Askiitians
 Buffer Solutions Math Methods Buffer Solution Mental Math
Buffer Solutions Math Methods Buffer Solution Mental Math
 Common Ion Effect Buffer Solution And Solubility Product Buffer Solution Solubility Solutions
Common Ion Effect Buffer Solution And Solubility Product Buffer Solution Solubility Solutions
 Introduction To Buffers Chemistry Libretexts
Introduction To Buffers Chemistry Libretexts
 What Is Solubility Solubility Product Solubility 11th Chemistry Electron Configuration
What Is Solubility Solubility Product Solubility 11th Chemistry Electron Configuration
 Buffer Solution Ph Calculations Video Khan Academy
Buffer Solution Ph Calculations Video Khan Academy
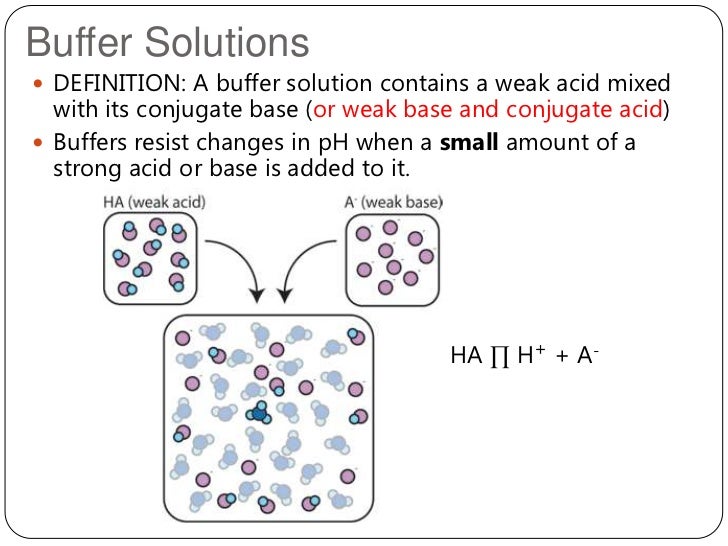 2012 Topic 18 2 Buffer Solutions
2012 Topic 18 2 Buffer Solutions
 A A Diagram Of The Process Of Agarose Gel Electrophoresis 1 An Agarose And Buffer Solution Is Heated And Poured Microbiology Study Chemistry Study Biology
A A Diagram Of The Process Of Agarose Gel Electrophoresis 1 An Agarose And Buffer Solution Is Heated And Poured Microbiology Study Chemistry Study Biology
 Buffer Solution Its Characteristics Types And Preparations
Buffer Solution Its Characteristics Types And Preparations
 A Buffered Vs A Non Buffered Solution Chem13 News Magazine University Of Waterloo
A Buffered Vs A Non Buffered Solution Chem13 News Magazine University Of Waterloo


