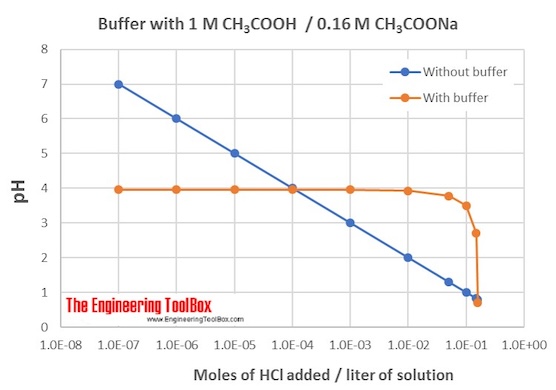
Standard Definition Of Buffer Solution
Buffer capacity is the amount of acid or base that can be added before the pH of a buffer changes. This is important for processes andor reactions which require specific and stable pH ranges.
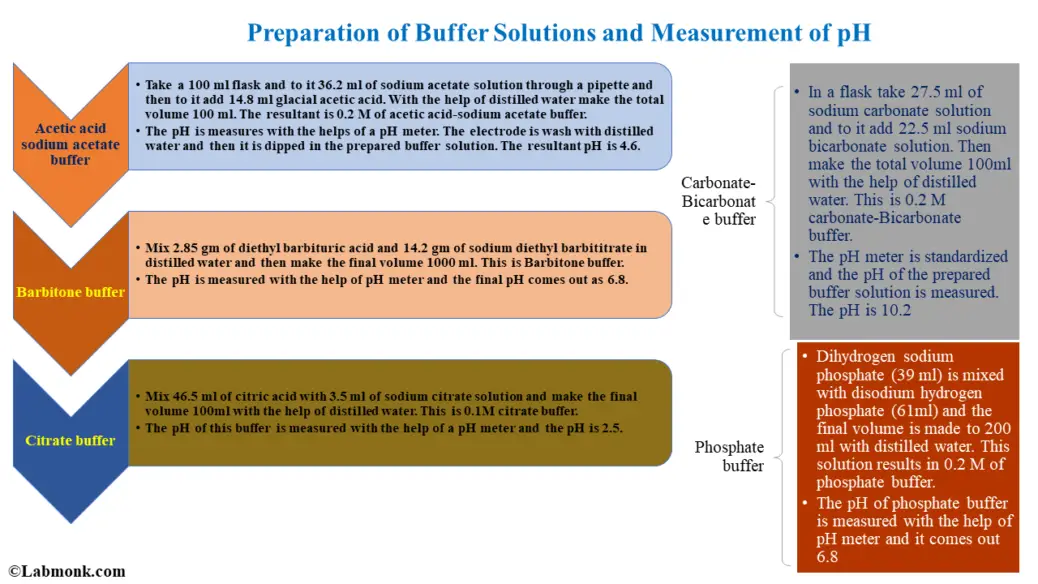 Preparation Of Buffer Solutions And Measurement Of Ph Labmonk
Preparation Of Buffer Solutions And Measurement Of Ph Labmonk
Its pH changes very little when a small amount of strong acid or base is added to it and thus it is used to prevent changes in the pH of a solution.

Standard definition of buffer solution. Buffer solutions are used for a wide range of chemical applications. This solution is quite important in the field of chemistry. Acidic buffer solutions are commonly made from a weak acid and one of its salts - often a sodium salt.
A calibration or buffer solution is a chemical solution that is used to calibrate a pH meter. Acidic buffer solutions are commonly made from a weak acid and one of its salts - often a sodium salt. A buffer is a solution that can resist pH change upon the addition of an acidic or basic components.
A buffer solution is one which resists changes in pH when small quantities of an acid or an alkali are added to it. Buffer solution and its types I basic buffer I acidic buffer I simple buffer I mixed buffer I by Chemistry by Dr. Buffer Solution is a water solvent based solution which consists of a mixture containing a weak acid and the conjugate base of the weak acid or a weak base and the conjugate acid of the weak base.
An acceptable value is obtained from the Control Standard measurement then the analysts can have improved confidence. The ability to relate measurements back to a stated reference usually an international standard. You can explore more about buffer solutions here.
What is a Buffer Solution. A buffer is an aqueous solution containing a weak acid and its conjugate base or a weak base and its conjugate acid. A buffer is an aqueous solution used to keep the pH of a solution nearly constant.
An acidic buffer solution is simply one which has a pH less than 7. A buffer solution is one that resists changes in pH when small amounts of acid or alkali are mixed with the buffer. Buffer solution A buffer is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid.
Furthermore it consists of a mixture of a weak acid and its conjugate base or vice-versa. It is able to neutralize small amounts of added acid or base thus maintaining the pH of the solution relatively stable. A solution which resists the change in its pH value even on the addition of a small amount of strong acid or base is called a buffer solution or buffer.
They resist a change in pH upon dilution or upon the addition of small amounts of acidalkali to them. Buffers are extremely useful in these systems to maintain the pH at a constant value. Free ebooks are available on every different subject you can think of in both fiction and non-fiction.
In other words a buffer is a solution that is able to maintain the pH condition of a solution. Buffers usually consist of a weak acid and its conjugate base in relatively equal and large quantities. Buffer Solution pH 100 002 20C 10105.
Mixture of acetic acid CH 3 COOH and Sodium acetate CH 3 COONa in water. It is used to prevent any change in the pH of a solution regardless of solute. This buffer solution definition as one of the most full of zip sellers here will completely be in the midst of the best options to review.
Reagecons pH buffer standards meet the ISO definition of traceability. A buffer solution is a solution containing weak acids and their conjugate bases or weak bases and conjugated acids that are resistant to pH changes. A buffer solution is one in which the pH of the solution is resistant to small additions of either a strong acid or strong base.
Buffer Solutions Buffers are solutions that resist a change in pH on dilution or on addition of small amounts of acids or alkali. A lot of biological and chemical reactions need a constant pH for the reaction to proceed. Its pH changes very little when a small amount of strong acid or base is added to it and is thus used to prevent a solution s pH change.
A buffer solution more precisely pH buffer or hydrogen ion buffer is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or vice versa. Its pH changes very little when a small amount of strong acid or base is added to it. There are free ebooks available for adults and kids and even those tween and teenage readers.
A buffer solution refers to an aqueous solution. It has a definite pH value. Suresh Thakur 2 years ago 4 minutes 57 seconds 2792 views This video is meant to strengthen the basics of chemistry.
An example of a buffer solution is bicarbonate in blood which maintains the bodys internal pH. A buffer solution more precisely pH buffer or hydrogen ion buffer is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or vice versa. A buffers pH changes very little when a small amount of strong acid or base is added to it.
A buffer is an aqueous solution that consists of a mixture of a weak acid and its salt acid buffer or a weak base with its salt basic buffer. Its pH changes very little when a small amount of strong acid or base is added to it. A buffer consists of a weak acid and its conjugate base or a weak base and its conjugate acid.
 Buffer Solutions Math Methods Buffer Solution Mental Math
Buffer Solutions Math Methods Buffer Solution Mental Math
 A Buffered Vs A Non Buffered Solution Chem13 News Magazine University Of Waterloo
A Buffered Vs A Non Buffered Solution Chem13 News Magazine University Of Waterloo
 Buffer Solutions Definition Types Preparation Examples And Videos
Buffer Solutions Definition Types Preparation Examples And Videos
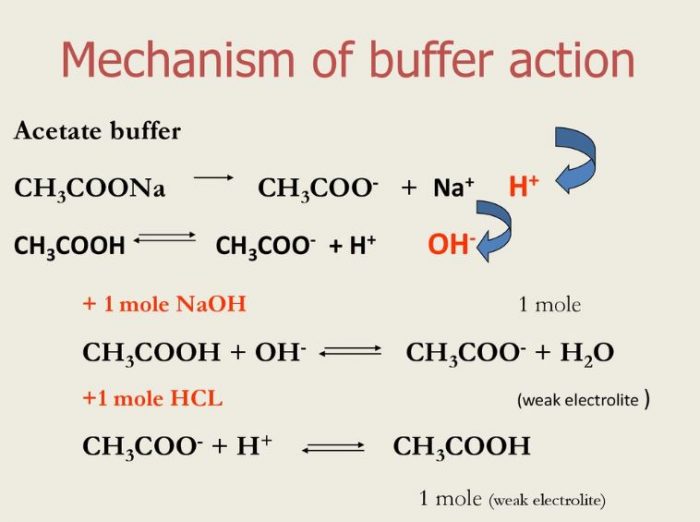 Buffer Solution And Buffer Action Chemistry Class 11 Ionic Equilibrium
Buffer Solution And Buffer Action Chemistry Class 11 Ionic Equilibrium
 What Is A Buffer And How Does It Work Westlab
What Is A Buffer And How Does It Work Westlab
 Buffer Solution Acidic Buffer Basic Buffer Animation Buffer Solution Electron Configuration Solutions
Buffer Solution Acidic Buffer Basic Buffer Animation Buffer Solution Electron Configuration Solutions
 Buffer Solution Ph Calculations Video Khan Academy
Buffer Solution Ph Calculations Video Khan Academy
 Common Ion Effect Buffer Solution And Solubility Product Buffer Solution Solubility Solutions
Common Ion Effect Buffer Solution And Solubility Product Buffer Solution Solubility Solutions
 Buffer Solution Preparation Of Buffer Solution Acidic Basic Buffer Buffer Action Buffer Solution Electron Configuration Solutions
Buffer Solution Preparation Of Buffer Solution Acidic Basic Buffer Buffer Action Buffer Solution Electron Configuration Solutions
 Buffer Solution Its Characteristics Types And Preparations
Buffer Solution Its Characteristics Types And Preparations
 Ph Chart For Acids And Bases Ph Chart Study Chemistry Electron Configuration
Ph Chart For Acids And Bases Ph Chart Study Chemistry Electron Configuration
 Common Ion Effect Animation Chemistry Ionic Equilibrium
Common Ion Effect Animation Chemistry Ionic Equilibrium
 Hydrogen Bonding Definition Examples And Types Digital Kemistry Hydrogen Bond Bond Molecules
Hydrogen Bonding Definition Examples And Types Digital Kemistry Hydrogen Bond Bond Molecules
 Solubility Common Ion Effect Buffer Solution Buffer Solution Solubility Solutions
Solubility Common Ion Effect Buffer Solution Buffer Solution Solubility Solutions